
If the initial concentration of butadiene was 0.985 M, what is the concentration after 1. Consider the following reaction of butadiene (C 4H 6) gas.Īt a given temperature, the reaction is second order and the half-life of butadiene is 236 seconds. If you measure two consecutive half-lives and they. If the initial concentration is 8.65 M, what is the concentration after 2.3 minutes? What is the half-life of NO 2?Įxercise 5. A second order concentration-time graph has an increasing half-life. The rate constant was determined to be 0.483 M -1s -1. Consider the following reaction at 250 oC. If the initial concentration of cyclobutane is 8.50 M, what is the concentration after 0.045 seconds?Įxercise 4. The decomposition of cyclobutane (C 4H 8) is a first order process. How long will it take for K-40 to decay to 20% of its initial concentration? All radioactive decay follows first order kinetics.Įxercise 3. The half-life for the radioactive decay of K-40 is 1.30 x 10 9 years. For a second-order reaction each half-life is twice as long as the life span of the one before. For a second-order reaction, the half-life is inversely related to the initial concentration of the reactant (A). If the experiment begins with a concentration of A equal to 0.25 M, how long, in s, will it take for the concentration of A to decrease to 0.047 M?Įxercise 2. The half life of any reaction is the amount of time it takes to consume half of the initial material. A reaction, A + B → P, has a rate constant of 3.2 x 10 -3 s -1. Use Half-Life to determine the Age of a Wooden RelicĮxercise 1. Worksheet: Integrated Rate Laws and Half-Life
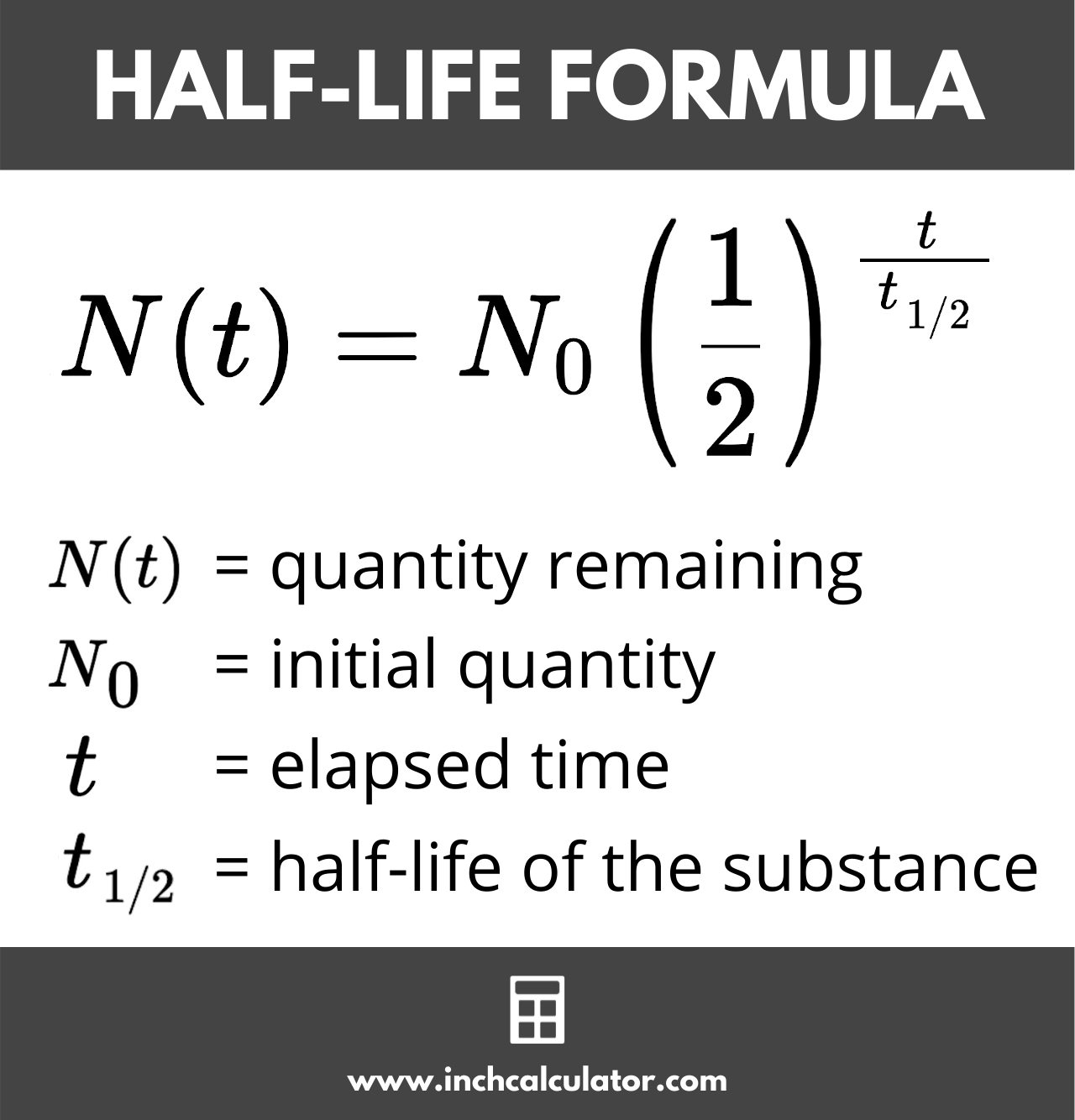
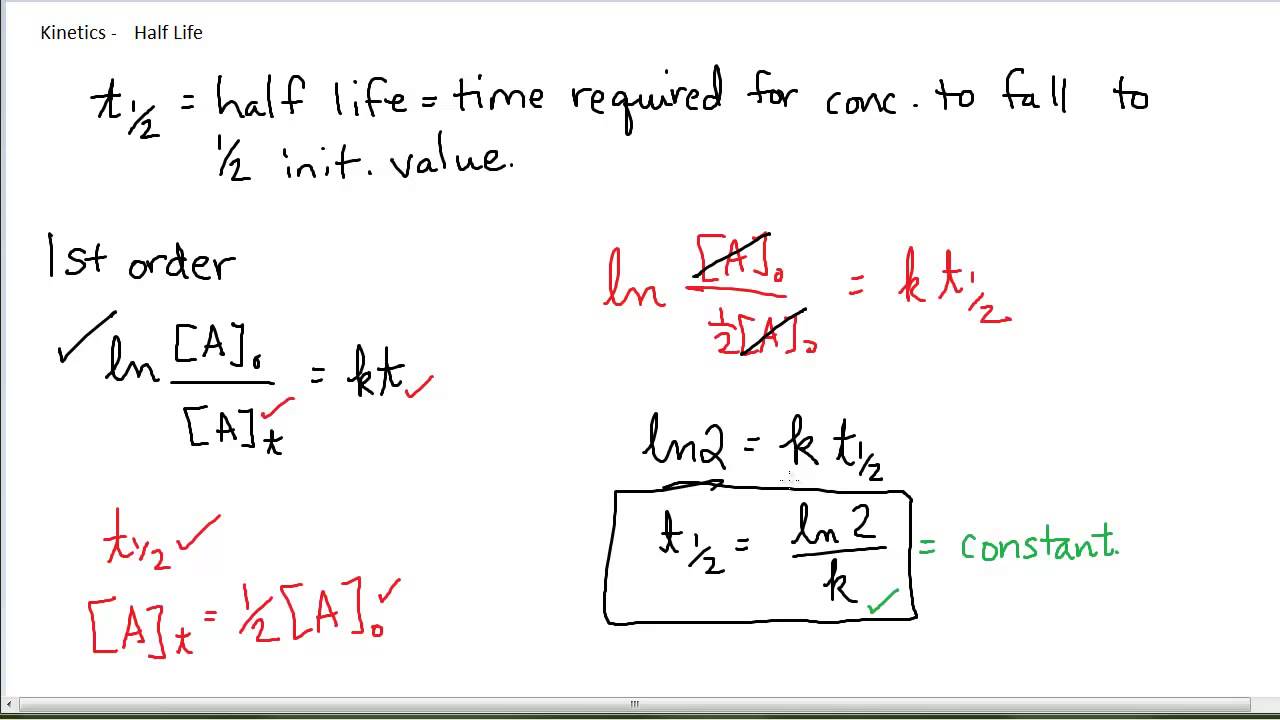
\(\displaystyle \mathrm\)įor a second order reaction, the half-life is inversely proportional to the initial concentration of the reactant and to k.īelow is a summary of integrated rate laws and half-lives for zero, first, and second order reactions. Here I will derive integrated rate laws for zero, first, and second order rate laws. The half-life of a second-order reaction given by:Integrated rate laws include time as a variable. The half-life of a second-order reaction is proportional to the square root of the rate constant. The rate of a second-order reaction is inversely proportional to the half-life. The first order half-life equation, t1/2 0.693/k. The half-life of a second-order reaction is inversely proportional to the rate constant. zero and second order notice the inclusion of AO in their half-life equations. The rate of a second-order reaction given by: Where is the concentration of the reactant. The half-life of second-order reactions is affected by both the initial concentration and the rate constant. For a second-order reaction, the half-life is inversely related to the initial concentration of the reactant (A). He half-life of a second-order reaction given by: Where t 1/2 is the half-life, ln is the natural logarithm, and k is the reaction rate constant. The half-life (t1/2) for any reaction is simply the amount of time required for exactly one half of the reactant to be consumed. The half-life can calculated using the following equation: The half-life of a second-order reaction is the time it takes for the reaction to reach half of its initial value. The reaction coordinate for this reaction is the transfer of an electron from one molecule of water to another molecule of water. This reaction catalyzed by the enzyme hydrogenase. One of the most common examples of a second-order reaction is the formation of hydrogen gas from water.This process known as a reaction coordinate. The molecules collide in such a way that their electrons rearrange to form a new molecule. A second-order reaction is a chemical reaction that occurs as a result of the collision of two molecules.In a second order reaction, the rate of the reaction is proportional to the concentration of the reactant raised to the power of two.


 0 kommentar(er)
0 kommentar(er)
